
A simple, sensitive, and straightforward LC-MS approach for rapid analysis of three potential genotoxic impurities in rabeprazole formulations.
Sign Up to like & getrecommendations! Published in 2018 at "Journal of separation science"
DOI: 10.1002/jssc.201800626
Abstract: In the present study, a sensitive and fully validated liquid chromatography with mass spectrometry method was developed for the quantification of three potential genotoxic impurities in rabeprazole drug substance. The separation was achieved on Symmetry… read more here.
Keywords: simple sensitive; potential genotoxic; impurities rabeprazole; three potential ... See more keywords

Detection of Two Genotoxic Impurities in Drug Substance and Preparation of Imatinib Mesylate by LC–MS/MS
Sign Up to like & getrecommendations! Published in 2020 at "Chromatographia"
DOI: 10.1007/s10337-020-03903-1
Abstract: Abstract As a potential DNA damaging substance, genotoxic impurities have been concerned by regulatory authorities in various countries. Two genotoxic impurities were found in imatinib mesylate which was a classical small molecule inhibitor of tyrosine… read more here.
Keywords: two genotoxic; substance; drug; imatinib mesylate ... See more keywords

Chemometrically assisted development and validation of LC–MS/MS method for the analysis of potential genotoxic impurities in meropenem active pharmaceutical ingredient
Sign Up to like & getrecommendations! Published in 2017 at "Journal of Pharmaceutical and Biomedical Analysis"
DOI: 10.1016/j.jpba.2017.06.061
Abstract: Graphical abstract Figure. No Caption available. HighlightsNovel LC–MS/MS method for simultaneous determination of 3 PGIs in meropenem API.A Box‐Behnken design was utilized for method development.Grid point search methodology was implemented aiming to obtain the optimal… read more here.
Keywords: methodology; potential genotoxic; analysis; method ... See more keywords
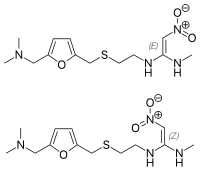
Determination of potentially genotoxic impurities in crotamiton active pharmaceutical ingredient by gas chromatography.
Sign Up to like & getrecommendations! Published in 2021 at "Journal of pharmaceutical and biomedical analysis"
DOI: 10.1016/j.jpba.2021.114544
Abstract: In the recent years control of potentially genotoxic impurities have an increasing importance in the analysis of active pharmaceutical ingredients. Guidelines of different regulatory bodies specify very low limits for these impurities, in many cases… read more here.
Keywords: genotoxic impurities; gas; active pharmaceutical; impurities crotamiton ... See more keywords

Molecularly imprinted polymer embedded-cryogels as selective genotoxic impurity scavengers
Sign Up to like & getrecommendations! Published in 2021 at "Separation Science and Technology"
DOI: 10.1080/01496395.2020.1869259
Abstract: ABSTRACT This research aims to design, preparation, and characterization of molecularly imprinted polymeric particles embedded-cryogels for the selective removal of potentially genotoxic impurities 2-aminopyridine and 5-amino-2-chloropyridine in model active pharmaceutical ingredients piroxicam and tenoxicam. For… read more here.
Keywords: potentially genotoxic; particles embedded; molecularly imprinted; cryogels selective ... See more keywords

Development and Validation of a Sensitive GC-MS/MS Method for the Determination of Five Potential Genotoxic Impurities in Abiraterone Acetate.
Sign Up to like & getrecommendations! Published in 2021 at "Journal of chromatographic science"
DOI: 10.1093/chromsci/bmab052
Abstract: A sensitive and selective gas chromatography-tandem mass spectrometry method was developed for the identification and quantification of five potential genotoxic impurities (PGIs), i.e., chloromethane, 2-chloropropane, 2-bromopropane, 4-chloro-1-butanol and diethyl sulfate, in abiraterone acetate. The method… read more here.
Keywords: potential genotoxic; abiraterone acetate; method; five potential ... See more keywords

UHPLC-MS/MS Method Development and Validation for the Genotoxic Impurities Trimethyl Phosphate and Triisopropyl Phosphate of Elagolix Sodium.
Sign Up to like & getrecommendations! Published in 2023 at "Journal of chromatographic science"
DOI: 10.1093/chromsci/bmad038
Abstract: Elagolix sodium is a gonadotropin-releasing hormone (GnRH) receptor antagonist that inhibits endogenous GnRH signaling by competitively binding to GnRH receptors in the pituitary gland to treat moderate to severe pain associated with endometriosis. To keep… read more here.
Keywords: triisopropyl phosphate; phosphate triisopropyl; elagolix sodium; genotoxic impurities ... See more keywords

A Validated GC-MS Method for the Determination of Genotoxic Impurities in Divalproex Sodium Drug Substance.
Sign Up to like & getrecommendations! Published in 2019 at "Journal of chromatographic science"
DOI: 10.1093/chromsci/bmy089
Abstract: A specific GC-MS method has been developed, optimized and validated for the determination of five genotoxic impurities namely Methyl bromide (Me.-Br), Ethyl bromide (Et.-Br), Isopropyl bromide (Ipr.-Br), n-Propyl bromide (n-Pr.-Br) and n-Butyl bromide (n-But.-Br) in… read more here.
Keywords: determination; drug substance; divalproex sodium; genotoxic impurities ... See more keywords