
An in vitro system for measuring genotoxicity mediated by human CYP3A4 in Saccharomyces cerevisiae
Sign Up to like & getrecommendations! Published in 2017 at "Environmental and Molecular Mutagenesis"
DOI: 10.1002/em.22093
Abstract: P450 activity is required to metabolically activate many chemical carcinogens, rendering them highly genotoxic. CYP3A4 is the most abundantly expressed P450 enzyme in the liver, accounting for most drug metabolism and constituting 50% of all… read more here.
Keywords: saccharomyces cerevisiae; genotoxicity; human cyp3a4; yeast ... See more keywords

The in vitro genotoxicity potency of mixtures of pyrrolizidine alkaloids can be explained by dose addition of the individual mixture components
Sign Up to like & getrecommendations! Published in 2022 at "Environmental and Molecular Mutagenesis"
DOI: 10.1002/em.22512
Abstract: Plant‐based 1,2‐unsaturated Pyrrolizidine Alkaloids (PAs) are responsible for liver genotoxicity/carcinogenicity following metabolic activation, making them a relevant concern for safety assessment. Due to 21st century toxicology approaches, risk of PAs can be better discerned though… read more here.
Keywords: potency; vitro genotoxicity; genotoxic potency; genotoxicity potency ... See more keywords

Establishing a quantitative framework for regulatory interpretation of genetic toxicity dose–response data: Margin of exposure case study of 48 compounds with both in vivo mutagenicity and carcinogenicity dose–response data
Sign Up to like & getrecommendations! Published in 2022 at "Environmental and Molecular Mutagenesis"
DOI: 10.1002/em.22517
Abstract: Quantitative relationships between carcinogenic potency and mutagenic potency have been previously examined using a benchmark dose (BMD)‐based approach. We extended those analyses by using human exposure data for 48 compounds to calculate carcinogenicity‐derived and genotoxicity‐derived… read more here.
Keywords: carcinogenicity; response data; dose response; genotoxicity ... See more keywords

Quantitative in vitro to in vivo extrapolation of genotoxicity data provides protective estimates of in vivo dose
Sign Up to like & getrecommendations! Published in 2022 at "Environmental and Molecular Mutagenesis"
DOI: 10.1002/em.22521
Abstract: Genotoxicity assessment is a critical component in the development and evaluation of chemicals. Traditional genotoxicity assays (i.e., mutagenicity, clastogenicity, and aneugenicity) have been limited to dichotomous hazard classification, while other toxicity endpoints are assessed through… read more here.
Keywords: genotoxicity data; genotoxicity; vitro vivo; vivo extrapolation ... See more keywords

Assessing the Quality and Making Appropriate Use of Historical Negative Control Data: A Report of the International Workshop on Genotoxicity Testing (IWGT).
Sign Up to like & getrecommendations! Published in 2023 at "Environmental and molecular mutagenesis"
DOI: 10.1002/em.22541
Abstract: Historical negative control data (HCD) have played an increasingly important role in interpreting the results of genotoxicity tests. In particular, Organisation for Economic Co-operation and Development (OECD) genetic toxicology Test Guidelines recommend comparing responses produced… read more here.
Keywords: genotoxicity testing; hcd; genotoxicity; negative control ... See more keywords

The comet assay in Folsomia candida: A suitable approach to assess genotoxicity in collembolans.
Sign Up to like & getrecommendations! Published in 2017 at "Environmental toxicology and chemistry"
DOI: 10.1002/etc.3795
Abstract: The present study shows the comet assay technique being successfully applied for the first time to one of the most widely used soil organisms in standardized ecotoxicological tests, Folsomia candida, providing a step forward in… read more here.
Keywords: comet assay; methodology; folsomia candida; assay folsomia ... See more keywords

In vitro genotoxicity assessment of an oxidized dextrin‐based hydrogel for biomedical applications
Sign Up to like & getrecommendations! Published in 2019 at "Journal of Applied Toxicology"
DOI: 10.1002/jat.3754
Abstract: Hydrogels are three‐dimensional, crosslinked networks of hydrophilic polymers swollen with a large amount of water or biological fluids, without dissolving. Dextrin, a low‐molecular‐weight carbohydrate composed by glucose residues, has been used to develop an injectable… read more here.
Keywords: based hydrogel; hydrogel biomedical; genotoxicity; dextrin based ... See more keywords

General toxicity and genotoxicity of altertoxin I: A novel 28‐day multiendpoint assessment in male Sprague–Dawley rats
Sign Up to like & getrecommendations! Published in 2022 at "Journal of Applied Toxicology"
DOI: 10.1002/jat.4297
Abstract: The mycotoxin altertoxin I (ATX‐I) is one of secondary metabolites produced by Alternaria fungi and is frequently detected as food and feed contaminants. Little is known about the genotoxicity of the ATX‐I. In order to… read more here.
Keywords: sprague dawley; novel day; day multiendpoint; male sprague ... See more keywords
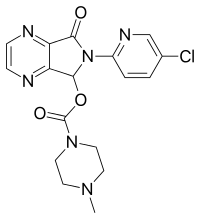
Neonatal subchronic toxicity and in vitro genotoxicity studies of the human‐identical milk oligosaccharide 3‐fucosyllactose
Sign Up to like & getrecommendations! Published in 2022 at "Journal of Applied Toxicology"
DOI: 10.1002/jat.4335
Abstract: Human milk oligosaccharides, such as 3‐fucosyllactose (3‐FL), are bioactive components of breast milk associated with benefits for infant growth and development. Structurally identical compounds (human‐identical milk oligosaccharides—HiMOs) can be produced using microbial fermentation, allowing their… read more here.
Keywords: day; subchronic toxicity; human identical; identical milk ... See more keywords

Safety evaluation of Veillonella atypica FB0054 with genotoxicity and subchronic toxicological studies
Sign Up to like & getrecommendations! Published in 2022 at "Journal of Applied Toxicology"
DOI: 10.1002/jat.4426
Abstract: Veillonella atypica is a nonmotile, nonsporulating anaerobic bacteria commonly found in various human biofilms. V. atypica FB0054 was isolated from the gastrointestinal tract of marathon runners, who have increased amounts of this species after athletic… read more here.
Keywords: evaluation; safety; atypica; subchronic toxicological ... See more keywords

Genotoxicity testing of nanomaterials.
Sign Up to like & getrecommendations! Published in 2022 at "Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology"
DOI: 10.1002/wnan.1833
Abstract: Nanomaterials have outstanding and unprecedented advantageous material properties but may also cause adverse effects in humans upon exposure. Testing nanomaterials for genotoxic properties is challenging because traditional testing methods were designed for small, soluble molecules… read more here.
Keywords: toxicology; issues nanomedicine; genotoxicity; mutation tests ... See more keywords