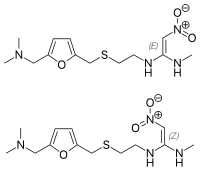
Detection and Characterization of an Unknown Impurity in Levothyroxine Oral Solution Product: Implications for Formulation Development and Storage.
Sign Up to like & getrecommendations! Published in 2020 at "Journal of pharmaceutical sciences"
DOI: 10.1016/j.xphs.2020.09.054
Abstract: An existing USP(2010) impurity method for levothyroxine drug substance was modified to expand its applicability for the analysis of levothyroxine oral solution (OS) formulation while achieving desirable resolution between the components of OS formulation. When… read more here.
Keywords: oral solution; levothyroxine oral; formulation; unknown impurity ... See more keywords