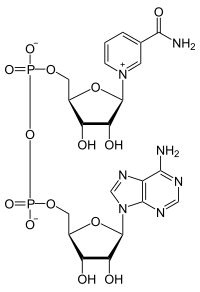
Incorporation of an Asymmetric Mo−Fe−S Cluster as an Artificial Cofactor into Nitrogenase
Sign Up to like & getrecommendations! Published in 2022 at "ChemBioChem"
DOI: 10.1002/cbic.202200384
Abstract: Nitrogenase employs a sophisticated electron transfer system and a Mo−Fe−S−C cofactor, designated the M‐cluster [(cit)MoFe7S9C]), to reduce atmospheric N2 to bioaccessible NH3. Previously, we have shown that the cofactor‐free form of nitrogenase can be repurposed… read more here.
Keywords: artificial cofactor; nitrogenase; cofactor; cluster ... See more keywords

NifA is the master regulator of both nitrogenase systems in Rhodobacter capsulatus
Sign Up to like & getrecommendations! Published in 2019 at "MicrobiologyOpen"
DOI: 10.1002/mbo3.921
Abstract: Rhodobacter capsulatus fixes atmospheric nitrogen (N2) by a molybdenum (Mo)‐nitrogenase and a Mo‐free iron (Fe)‐nitrogenase, whose production is induced or repressed by Mo, respectively. At low nanomolar Mo concentrations, both isoenzymes are synthesized and contribute… read more here.
Keywords: capsulatus; rhodobacter capsulatus; nifa master; rpon ... See more keywords

Aerobic nitrogen-fixing bacteria for hydrogen and ammonium production: current state and perspectives
Sign Up to like & getrecommendations! Published in 2019 at "Applied Microbiology and Biotechnology"
DOI: 10.1007/s00253-019-10210-9
Abstract: Biological nitrogen fixation (BNF) is accomplished through the action of the oxygen-sensitive enzyme nitrogenase. One unique caveat of this reaction is the inclusion of hydrogen gas (H2) evolution as a requirement of the reaction mechanism.… read more here.
Keywords: nitrogen; fixing bacteria; nitrogen fixing; hydrogen ... See more keywords
Structural characterization of the nitrogenase molybdenum-iron protein with the substrate acetylene trapped near the active site.
Sign Up to like & getrecommendations! Published in 2018 at "Journal of inorganic biochemistry"
DOI: 10.1016/j.jinorgbio.2017.12.008
Abstract: The biological reduction of dinitrogen (N2) to ammonia is catalyzed by the complex metalloenzyme nitrogenase. Structures of the nitrogenase component proteins, Iron (Fe) protein and Molybdenum‑iron (MoFe) protein, and the stabilized complexes these component proteins,… read more here.
Keywords: protein; active site; nitrogenase; iron ... See more keywords

Revealing a role for the G subunit in mediating interactions between the nitrogenase component proteins.
Sign Up to like & getrecommendations! Published in 2020 at "Journal of inorganic biochemistry"
DOI: 10.1016/j.jinorgbio.2020.111273
Abstract: Azotobacter vinelandii contains three forms of nitrogenase known as the Mo-, V-, and Fe-nitrogenases. They are all two-component enzyme systems, where the catalytic component, referred to as NifDK, VnfDGK, and AnfDGK, associates with the reductase… read more here.
Keywords: role subunit; nitrogenase; revealing role; subunit ... See more keywords

Mechanistic Insights into Nitrogenase FeMo-Cofactor Catalysis through a Steady-State Kinetic Model.
Sign Up to like & getrecommendations! Published in 2022 at "Biochemistry"
DOI: 10.1021/acs.biochem.2c00415
Abstract: Mo-nitrogenase catalyzes the challenging N2-to-NH3 reduction. This complex reaction proceeds through a series of intermediate states (En) of its active site FeMo-cofactor. An understanding of the kinetics of the conversion between En states is central… read more here.
Keywords: rate; state; nitrogenase; femo cofactor ... See more keywords

In Vivo Temperature Dependency of Molybdenum and Vanadium Nitrogenase Activity in the Heterocystous Cyanobacteria Anabaena variabilis.
Sign Up to like & getrecommendations! Published in 2022 at "Environmental science & technology"
DOI: 10.1021/acs.est.1c05279
Abstract: The reduction of atmospheric dinitrogen by nitrogenase is a key component of terrestrial nitrogen cycling. Nitrogenases exist in several isoforms named after the metal present within their active center: the molybdenum (Mo), the vanadium (V),… read more here.
Keywords: heterocystous cyanobacteria; nitrogenase; molybdenum vanadium; temperature ... See more keywords

The One-Electron Reduced Active-Site FeFe-Cofactor of Fe-Nitrogenase Contains a Hydride Bound to a Formally Oxidized Metal-Ion Core.
Sign Up to like & getrecommendations! Published in 2022 at "Inorganic chemistry"
DOI: 10.1021/acs.inorgchem.2c00180
Abstract: The nitrogenase active-site cofactor must accumulate 4e-/4H+ (E4(4H) state) before N2 can bind and be reduced. Earlier studies demonstrated that this E4(4H) state stores the reducing-equivalents as two hydrides, with the cofactor metal-ion core formally… read more here.
Keywords: metal ion; state; nitrogenase; cofactor ... See more keywords

QM/MM Study of Partial Dissociation of S2B for the E2 Intermediate of Nitrogenase
Sign Up to like & getrecommendations! Published in 2022 at "Inorganic Chemistry"
DOI: 10.1021/acs.inorgchem.2c02488
Abstract: Nitrogenase is the only enzyme that can cleave the triple bond in N2, making nitrogen available for all lifeforms. Previous computational studies have given widely diverging results regarding the reaction mechanism of the enzyme. For… read more here.
Keywords: s2b; study partial; nitrogenase; s2b intermediate ... See more keywords

A VTVH MCD and EPR Spectroscopic Study of the Maturation of the "Second" Nitrogenase P-Cluster.
Sign Up to like & getrecommendations! Published in 2018 at "Inorganic chemistry"
DOI: 10.1021/acs.inorgchem.8b00428
Abstract: The P-cluster of the nitrogenase MoFe protein is a [ Fe8 S7] cluster that mediates efficient transfer of electrons to the active site for substrate reduction. Arguably the most complex homometallic FeS cluster found in… read more here.
Keywords: mcd epr; epr spectroscopic; spectroscopic study; nitrogenase ... See more keywords

Solvent Deuterium Isotope Effects of Substrate Reduction by Nitrogenase from Azotobacter vinelandii
Sign Up to like & getrecommendations! Published in 2022 at "Journal of the American Chemical Society"
DOI: 10.1021/jacs.2c07574
Abstract: The mechanism of nitrogenase, the enzyme responsible for biological nitrogen fixation, has been of great interest for understanding the catalytic strategy utilized to reduce dinitrogen to ammonia under ambient temperatures and pressures. The reduction mechanism… read more here.
Keywords: deuterium isotope; nitrogenase; isotope effects; isotope ... See more keywords