
Population Pharmacokinetic Modeling and Exposure‐Response Analysis for Aripiprazole Once Monthly in Subjects With Schizophrenia
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology in Drug Development"
DOI: 10.1002/cpdd.1022
Abstract: An intramuscular formulation of aripiprazole monohydrate dosed once monthly (AOM) was developed to address nonadherence with the approved oral tablets. A 3‐compartment linear population pharmacokinetic model for oral and AOM doses was developed; relative bioavailability… read more here.
Keywords: population pharmacokinetic; population; exposure response; metabolizer status ... See more keywords

Population Pharmacokinetic Modeling and Simulation of TV‐46000: A Long‐Acting Injectable Formulation of Risperidone
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology in Drug Development"
DOI: 10.1002/cpdd.1078
Abstract: TV‐46000 is a long‐acting subcutaneous antipsychotic that uses a novel copolymer drug delivery technology in combination with a well‐characterized molecule, risperidone, that is in clinical development as a treatment for schizophrenia. A population pharmacokinetic (PPK)… read more here.
Keywords: 46000 long; development; population pharmacokinetic; modeling simulation ... See more keywords
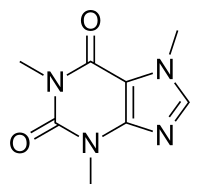
Population Pharmacokinetic/Pharmacodynamic Modeling of the Effect of Abrocitinib on QT Intervals in Healthy Volunteers
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology in Drug Development"
DOI: 10.1002/cpdd.1111
Abstract: Abrocitinib is a selective Janus kinase 1 inhibitor for the treatment of moderate to severe atopic dermatitis (AD). To assess the relationship between abrocitinib plasma concentrations and heart rate (HR)–corrected QT (QTc) and HR and… read more here.
Keywords: effect abrocitinib; healthy volunteers; effect; population pharmacokinetic ... See more keywords

Evaluation of Ethnicity Effect on Intranasal Esketamine Pharmacokinetics by Population Pharmacokinetic Modeling Using Data From a Japanese Phase 2b Study
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology in Drug Development"
DOI: 10.1002/cpdd.1214
Abstract: Esketamine is used for the treatment of treatment‐resistant depression in many countries. A population pharmacokinetic (popPK) model of esketamine and its metabolite (noresketamine) has been previously developed, which included Asian race and Japanese ethnicity as… read more here.
Keywords: ethnicity; population pharmacokinetic; esketamine; model ... See more keywords

Ropivacaine Wound Infiltration for Pain Management After Breast Cancer Mastectomy: A Population Pharmacokinetic Analysis
Sign Up to like & getrecommendations! Published in 2018 at "Clinical Pharmacology in Drug Development"
DOI: 10.1002/cpdd.452
Abstract: Ropivacaine continuous wound infusions (CWIs) are extensively used as a component of multimodal analgesia. The rational application of CWI of ropivacaine requires a thorough understanding of its pharmacokinetics to investigate the risk of potential systemic… read more here.
Keywords: ropivacaine wound; analysis; population pharmacokinetic; ropivacaine ... See more keywords

Population Pharmacokinetic Modeling and Probability of Pharmacodynamic Target Attainment for Ceftazidime-Avibactam in Pediatric Patients Aged 3 Months and Older.
Sign Up to like & getrecommendations! Published in 2021 at "Clinical pharmacology and therapeutics"
DOI: 10.1002/cpt.2460
Abstract: Increasing prevalence of infections caused by antimicrobial-resistant Gram-negative bacteria represents a global health crisis, and while several novel therapies that target various aspects of antimicrobial resistance have been introduced in recent years, few are currently… read more here.
Keywords: ceftazidime avibactam; months older; population pharmacokinetic; avibactam pediatric ... See more keywords

Population Pharmacokinetic Models for Direct Oral Anticoagulants: A Systematic Review and Clinical Appraisal Using Exposure Simulation
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology and Therapeutics"
DOI: 10.1002/cpt.2649
Abstract: Available data have shown an association between direct oral anticoagulant (DOAC) plasma concentration and clinical, particularly bleeding, events. Factors that may influence DOAC plasma concentration are therefore the focus of particular attention. Population pharmacokinetic (PopPK)… read more here.
Keywords: plasma concentration; population pharmacokinetic; direct oral; doac plasma ... See more keywords
Population Pharmacokinetic Analysis of Dalteparin in Pediatric Patients With Venous Thromboembolism
Sign Up to like & getrecommendations! Published in 2020 at "Journal of Clinical Pharmacology"
DOI: 10.1002/jcph.1716
Abstract: This article describes the population pharmacokinetics (PK) of dalteparin in pediatric patients with venous thromboembolism (VTE). A prospective multicenter open‐label study was conducted in children who required anticoagulation for the treatment of VTE. The study… read more here.
Keywords: patients venous; dalteparin pediatric; population pharmacokinetic; venous thromboembolism ... See more keywords

A Population Pharmacokinetic Meta‐Analysis of Veliparib, a PARP Inhibitor, Across Phase 1/2/3 Trials in Cancer Patients
Sign Up to like & getrecommendations! Published in 2021 at "Journal of Clinical Pharmacology"
DOI: 10.1002/jcph.1875
Abstract: Veliparib (ABT‐888) is a poly(ADP‐ribose) polymerase inhibitor in development for the treatment of high‐grade ovarian cancer or BRCA‐mutated breast cancer in combination with carboplatin and paclitaxel. The population pharmacokinetics of veliparib were characterized using combined… read more here.
Keywords: phase; inhibitor; population pharmacokinetic; veliparib ... See more keywords

Pooled Population Pharmacokinetic Analyses of Venetoclax in Patients Across Indications and Healthy Subjects from Phase 1, 2 and 3 Clinical Trials.
Sign Up to like & getrecommendations! Published in 2023 at "Journal of clinical pharmacology"
DOI: 10.1002/jcph.2248
Abstract: Following the decade long clinical investigation, venetoclax has accrued PK data across multiple populations and widely-ranging demographics, intrinsic, and extrinsic factors. We leveraged these rich data to systematically characterize venetoclax PK and assess covariate effects… read more here.
Keywords: indications healthy; cyp3a; pooled population; inhibitor ... See more keywords

A population pharmacokinetic model taking into account protein binding for the sustained-release granule formulation of valproic acid in children with epilepsy
Sign Up to like & getrecommendations! Published in 2018 at "European Journal of Clinical Pharmacology"
DOI: 10.1007/s00228-018-2444-2
Abstract: PurposeThe objective of this work was to develop a population pharmacokinetic model for a prolonged-release granule formulation of valproic acid (VPA) in children with epilepsy and to determine the doses providing a VPA trough concentration… read more here.
Keywords: release granule; pharmacokinetic model; granule formulation; formulation valproic ... See more keywords