
Real-world safety and effectiveness of nivolumab for advanced renal cell carcinoma in Japan: a post-marketing surveillance
Sign Up to like & getrecommendations! Published in 2022 at "International Journal of Clinical Oncology"
DOI: 10.1007/s10147-022-02155-3
Abstract: This all-case post-marketing surveillance (PMS) evaluated the real-world safety and effectiveness of nivolumab monotherapy in Japanese patients with un-resectable or metastatic renal cell carcinoma (RCC). This multicenter, open-label, non-interventional, observational PMS study (registered from August… read more here.
Keywords: safety effectiveness; post marketing; marketing surveillance; real world ... See more keywords

Safety and effectiveness of oral blonanserin for schizophrenia: A review of Japanese post-marketing surveillances.
Sign Up to like & getrecommendations! Published in 2021 at "Journal of pharmacological sciences"
DOI: 10.1016/j.jphs.2020.09.006
Abstract: Schizophrenia significantly limits social functioning with positive and negative symptoms and cognitive dysfunction. Blonanserin (LONASEN®), a novel second-generation antipsychotic approved for treating schizophrenia in Japan in 2008, reportedly shows beneficial effects on cognitive function as… read more here.
Keywords: schizophrenia; japanese post; post marketing; blonanserin ... See more keywords

Guidance for the governance of public-private collaborations in vaccine post-marketing settings in Europe.
Sign Up to like & getrecommendations! Published in 2019 at "Vaccine"
DOI: 10.1016/j.vaccine.2019.04.073
Abstract: INTRODUCTION The 2009 influenza pandemic highlighted challenges for vaccine post-marketing monitoring in Europe, particularly the need to have appropriate infrastructures to strengthen public-private collaborations (PPCs) with suitable processes to improve stakeholder interactions and collection and… read more here.
Keywords: public private; post marketing; governance; vaccine post ... See more keywords

Post-marketing safety surveillance for inactivated Enterovirus 71 vaccines in Jiangsu, China from 2017 to 2019.
Sign Up to like & getrecommendations! Published in 2021 at "Vaccine"
DOI: 10.1016/j.vaccine.2021.01.048
Abstract: INTRODUCTION Two types of enterovirus 71 (EV71) vaccines, manufactured using human diploid (H2) and Vero cells, have been administered in Jiangsu Province, China since 2017. In this study, we evaluated their safety profiles using records… read more here.
Keywords: safety; marketing safety; post marketing; china ... See more keywords

Generating comparative evidence on new drugs and devices after approval
Sign Up to like & getrecommendations! Published in 2020 at "The Lancet"
DOI: 10.1016/s0140-6736(19)33177-0
Abstract: Certain limitations of evidence available on drugs and devices at the time of market approval often persist in the post-marketing period. Often, post-marketing research landscape is fragmented. When regulatory agencies require pharmaceutical and device manufacturers… read more here.
Keywords: marketing; drugs devices; approval; post marketing ... See more keywords

Safety of the Xuesaitong injection in China: results from a large-scale multicentre post-marketing surveillance study in a real-world setting
Sign Up to like & getrecommendations! Published in 2020 at "Current Medical Research and Opinion"
DOI: 10.1080/03007995.2020.1832056
Abstract: Abstract Objective The safety profile of traditional Chinese medicine injections has emerged as the greatest challenge to their clinical application. The authors aimed to perform a post-marketing surveillance study in a real-world setting to evaluate… read more here.
Keywords: safety; study; post marketing; post ... See more keywords

Safety and efficacy of long-term treatment with teneligliptin: Interim analysis of a post-marketing surveillance of more than 10,000 Japanese patients with type 2 diabetes mellitus
Sign Up to like & getrecommendations! Published in 2018 at "Expert Opinion on Pharmacotherapy"
DOI: 10.1080/14656566.2017.1420165
Abstract: ABSTRACT Background: This post-marketing surveillance examined the safety and efficacy of long-term teneligliptin therapy in Japanese patients. Research design and methods: We report interim results (cut-off date: 28 June 2017) of a 3-year PMS undertaken… read more here.
Keywords: analysis; safety efficacy; safety; japanese patients ... See more keywords

Safety and effectiveness of empagliflozin according to body mass index in Japanese patients with type 2 diabetes: a subgroup analysis of a 3-year post-marketing surveillance study
Sign Up to like & getrecommendations! Published in 2022 at "Expert Opinion on Drug Safety"
DOI: 10.1080/14740338.2022.2062322
Abstract: ABSTRACT Background Empagliflozin, a glucose-lowering drug licensed for type 2 diabetes (T2D), demonstrated tolerability and effectiveness overall in a post-marketing surveillance (PMS) study in Japan. However, the impact of body mass index (BMI) is unclear.… read more here.
Keywords: body mass; marketing surveillance; type diabetes; post marketing ... See more keywords
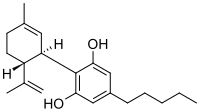
Post Marketing Safety of Plus CBD™ Products, a Full Spectrum Hemp Extract: A 2-Year Experience
Sign Up to like & getrecommendations! Published in 2020 at "Journal of Dietary Supplements"
DOI: 10.1080/19390211.2020.1767255
Abstract: Abstract The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured… read more here.
Keywords: hemp; spectrum hemp; year; safety ... See more keywords

Vedolizumab use in patients with inflammatory bowel diseases undergoing surgery: clinical trials and post-marketing experience
Sign Up to like & getrecommendations! Published in 2019 at "Gastroenterology Report"
DOI: 10.1093/gastro/goz034
Abstract: Abstract Background Patients with inflammatory bowel diseases frequently require surgery, but immunotherapies used in disease management may increase the risk of post-operative complications. We investigated frequencies of post-operative complications in patients who received vedolizumab—a gut-selective… read more here.
Keywords: surgery; bowel; post marketing; post operative ... See more keywords

Post-marketing surveillance study of trifluridine/tipiracil in patients with metastatic colorectal cancer.
Sign Up to like & getrecommendations! Published in 2021 at "Japanese journal of clinical oncology"
DOI: 10.1093/jjco/hyaa243
Abstract: BACKGROUND The novel oral nucleoside antineoplastic agent trifluridine/tipiracil was approved for metastatic colorectal cancer in Japan in March 2014. In this post-marketing surveillance study, we investigated the safety and efficacy of trifluridine/tipiracil in a real-world… read more here.
Keywords: metastatic colorectal; trifluridine tipiracil; tipiracil; colorectal cancer ... See more keywords