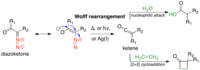
Synthesis of the hexacyclic triterpene core of the jujuboside saponins via tandem Wolff rearrangement-intramolecular ketene hetero-Diels-Alder reaction.
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron"
DOI: 10.1016/j.tet.2018.04.051
Abstract: The jujubosides are saponin natural products reported to have immunoadjuvant, anticancer, antibacterial, antifungal, and antisweet activities. The triterpene component, jujubogenin contains a unique tricyclic ketal motif comprising the DEF ring system. Herein, we describe our… read more here.
Keywords: diels alder; wolff rearrangement; tandem wolff; rearrangement intramolecular ... See more keywords

Diastereoselective, Multicomponent Synthesis of Pyrrolopyrazinoquinazolinones via a Tandem Quinazolinone Rearrangement/Intramolecular Ring Closure of Tautomeric (Z)-Benzamidines.
Sign Up to like & getrecommendations! Published in 2021 at "Organic letters"
DOI: 10.1021/acs.orglett.1c01955
Abstract: An expedient route to enantiopure, diastereomeric pyrrolopyrazinoquinazolinones was developed following the discovery of a domino quinazolinone rearrangement-intramolecular cyclization of N-H benzamidines. A Ugi-Mumm-Staudinger sequence employing an optically pure proline derivative gave quinazolinones that, upon N-Boc… read more here.
Keywords: quinazolinone rearrangement; multicomponent synthesis; rearrangement intramolecular; diastereoselective multicomponent ... See more keywords