
Synthesis and theoretical study of pyrrole formiate derivatives through ring contraction of 1,4-dihydropyridines
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron"
DOI: 10.1016/j.tet.2018.11.021
Abstract: Abstract Pyrrole formiate derivatives were synthesized through ring contraction of corresponding 3-carboxylate-1,4-dihydropyridines (3-CDHPs) mediated by 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO). 2-Carboxylate-3-aryl-1H-pyrroles (2) and 3-carboxylate-4-aryl-1H-pyrroles (3) were obtained under readily accessible reaction conditions. To gain deep insights into this… read more here.
Keywords: synthesis theoretical; formiate derivatives; pyrrole formiate; ring contraction ... See more keywords

Synthesis of cyclic olefins via Mitsunobu C-alkylation followed by Ramberg-Bäcklund ring contraction
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron Letters"
DOI: 10.1016/j.tetlet.2018.06.014
Abstract: Abstract Cyclic olefins were prepared via a novel synthetic approach that involves the formation of two C C bonds in a potentially stereoselective fashion. The first bond is formed by employing a Mitsunobu dehydrative C-alkylation;… read more here.
Keywords: olefins via; synthesis cyclic; cyclic olefins; ring contraction ... See more keywords

Ring Contraction of Tropylium Ions into Benzenoid Derivatives.
Sign Up to like & getrecommendations! Published in 2022 at "Organic letters"
DOI: 10.1021/acs.orglett.2c00663
Abstract: We report a method to convert substituted tropylium ions into benzenoid derivatives. read more here.
Keywords: contraction tropylium; ions benzenoid; benzenoid derivatives; tropylium ions ... See more keywords

Pd-Catalyzed Tandem [5 + 2] Cycloaddition/Ring Contraction of Phthalide-Derived Alkenes and Vinylethylene Carbonates for the Construction of Benzo-[5,5]-spiroketal Lactones.
Sign Up to like & getrecommendations! Published in 2022 at "Organic letters"
DOI: 10.1021/acs.orglett.2c01114
Abstract: An annulation of phthalide-derived activated alkenes is initially disclosed in this work. Specifically, we have developed an unprecedented [5 + 2] cycloaddition/ring-contraction tandem process of activated tetrasubstituted alkenes derived from phthalides or butyrolactone with vinylethylene… read more here.
Keywords: spiroketal lactones; vinylethylene carbonates; phthalide derived; benzo spiroketal ... See more keywords
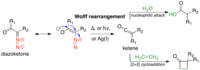
Unexpected Ring Contraction of Homophthalic Anhydrides under Diazo Transfer Conditions.
Sign Up to like & getrecommendations! Published in 2022 at "Organic letters"
DOI: 10.1021/acs.orglett.2c01730
Abstract: An attempted Regitz diazo transfer onto homophthalic anhydride led to the discovery of an unexpected ring contraction, which gave N-sulfonyl phthalide-3-carboxamide derivatives. The reaction is thought to proceed via a [3 + 2] cycloaddition of… read more here.
Keywords: ring contraction; diazo transfer; unexpected ring; contraction homophthalic ... See more keywords

Synthesis of Pyrroles via Consecutive 6π-Electrocyclization/Ring-Contraction of Sulfilimines
Sign Up to like & getrecommendations! Published in 2021 at "Journal of the American Chemical Society"
DOI: 10.1021/jacs.1c04835
Abstract: We present a modular, synthetic entry to polysubstituted pyrroles employing readily available 2,5-dihydrothiophenes. Ring-opening of the heterocycle provides access to a panel of 1,3-dienes which undergo pyrrole formation in the presence of inexpensive chloramine-T trihydrate.… read more here.
Keywords: ring contraction; synthesis pyrroles; via consecutive; contraction ... See more keywords

Deciphering the enzymatic mechanism of sugar ring contraction in UDP-apiose biosynthesis
Sign Up to like & getrecommendations! Published in 2019 at "Nature catalysis"
DOI: 10.1038/s41929-019-0382-8
Abstract: The C-branched pentose sugar d-apiose is important for plant cell wall development. Its biosynthesis as urdine diphosphate-d-apiose (UDP-d-apiose) involves decarboxylation of the UDP-d-glucuronic acid precursor coupled with pyranosyl-to-furanosyl sugar ring contraction. This unusual multistep reaction… read more here.
Keywords: sugar ring; biosynthesis; udp apiose; ring contraction ... See more keywords

Ring contraction in synthesis of functionalized carbocycles.
Sign Up to like & getrecommendations! Published in 2022 at "Chemical Society reviews"
DOI: 10.1039/d1cs01080h
Abstract: Carbocycles are a key and widely present structural motif in organic compounds. The construction of structurally intriguing carbocycles, such as highly-strained fused rings, spirocycles or highly-functionalized carbocycles with congested stereocenters, remains challenging in organic chemistry.… read more here.
Keywords: synthesis functionalized; chemistry; functionalized carbocycles; ring contraction ... See more keywords

N-Chlorinative Ring Contraction of 1,4-Dimethoxyphthalazines via a Bicyclization/Ring-Opening Mechanism
Sign Up to like & getrecommendations! Published in 2020 at "Synthesis"
DOI: 10.1055/s-0040-1706639
Abstract: An unprecedented N-chlorinative ring contraction of 1,2-diazines was discovered and investigated with an electrophilic chlorinating reagent, trichloroisocyanuric acid (TCICA). Through optimization and mechanistic analysis, the assisting role of n-Bu4NCl as an exogenous nucleophile was identified,… read more here.
Keywords: ring opening; opening mechanism; chlorinative ring; bicyclization ring ... See more keywords

Nucleophile-induced ring contraction in pyrrolo[2,1-c][1,4]benzothiazines: access to pyrrolo[2,1-b][1,3]benzothiazoles
Sign Up to like & getrecommendations! Published in 2023 at "Beilstein Journal of Organic Chemistry"
DOI: 10.3762/bjoc.19.46
Abstract: Pyrrolo[2,1-b][1,3]benzothiazoles are an important class of fused sulfur and nitrogen-containing heterocycles intensively studied in medicinal chemistry and pharmacology. In the present paper, a new synthetic approach to pyrrolobenzothiazoles is developed based on 1,4-thiazine ring contraction… read more here.
Keywords: pyrrolo; nucleophile induced; chemistry; pyrrolo benzothiazoles ... See more keywords