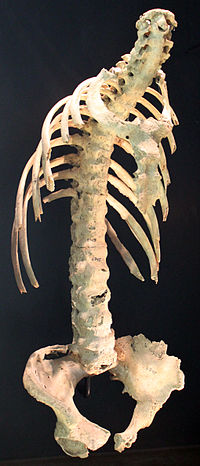
Secukinumab: A Review in Ankylosing Spondylitis
Sign Up to like & getrecommendations! Published in 2019 at "Drugs"
DOI: 10.1007/s40265-019-01075-3
Abstract: Secukinumab (Cosentyx®), a first-in-class fully human monoclonal antibody against interleukin-17A, is approved in several countries, including the USA and those of the EU, for the treatment of ankylosing spondylitis (AS). Subcutaneous secukinumab significantly improved the… read more here.
Keywords: ankylosing spondylitis; treatment; review ankylosing; secukinumab ... See more keywords

Interet du sécukinumab pour le traitement de la maladie de Verneuil
Sign Up to like & getrecommendations! Published in 2019 at "Annales De Dermatologie Et De Venereologie"
DOI: 10.1016/j.annder.2019.09.075
Abstract: Introduction La maladie de Verneuil (MV) est une maladie inflammatoire chronique avec un impact majeur sur la qualite de vie des patients atteints. Dans la MV, le taux d’IL-17 est augmente au niveau serique et in… read more here.
Keywords: maladie verneuil; traitement; pour traitement; secukinumab ... See more keywords

Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain
Sign Up to like & getrecommendations! Published in 2019 at "Journal of Dermatological Treatment"
DOI: 10.1080/09546634.2018.1528000
Abstract: Abstract Background: The efficacy and safety of secukinumab in patients with plaque psoriasis (PsO) have been demonstrated in randomized clinical trials (RCTs). However, data regarding its efficacy and safety in real-life settings are scarce. Objectives:… read more here.
Keywords: patients plaque; efficacy safety; treatment; secukinumab ... See more keywords

Efficacy, safety, and drug survival of patients with psoriasis treated with IL-17 inhibitors – brodalumab, ixekizumab, and secukinumab: real-world data from the Czech Republic BIOREP registry
Sign Up to like & getrecommendations! Published in 2022 at "Journal of Dermatological Treatment"
DOI: 10.1080/09546634.2022.2082354
Abstract: Abstract Background Real-world data on the use of interleukin-17 (IL-17) inhibitors for the treatment of psoriasis are limited. Objective To evaluate and compare the efficacy, safety, and drug survival of IL-17 inhibitors. Methods This retrospective… read more here.
Keywords: safety; secukinumab; drug survival; ixekizumab secukinumab ... See more keywords

Budget impact analysis of secukinumab versus adalimumab in the treatment of ankylosing spondylitis
Sign Up to like & getrecommendations! Published in 2019 at "Journal of Medical Economics"
DOI: 10.1080/13696998.2018.1551227
Abstract: Abstract Background: Biologic treatments have enhanced the treatment outcomes of patients with active ankylosing spondylitis (AS). Until recently, TNF-alpha-inhibitors have been the only biologics approved for the treatment of active AS. The objective of this… read more here.
Keywords: ankylosing spondylitis; budget impact; analysis; treatment ... See more keywords

Safety evaluation of secukinumab in pediatric patients with plaque psoriasis
Sign Up to like & getrecommendations! Published in 2022 at "Expert Opinion on Drug Safety"
DOI: 10.1080/14740338.2022.2073349
Abstract: ABSTRACT Introduction Psoriasis (Ps) is a common chronic, recurrent, immune-mediated, inflammatory skin disease affecting up to 2% of children. It has a well-established impact on patients’ quality of life. Moreover, patients with psoriasis exhibit a… read more here.
Keywords: safety; secukinumab; pediatric population; safety evaluation ... See more keywords

O19 Secukinumab-related gastrointestinal safety in PsA and AS
Sign Up to like & getrecommendations! Published in 2020 at "Rheumatology"
DOI: 10.1093/rheumatology/keaa110.018
Abstract: Secukinumab is a selective interleukin-17a inhibitor (anti-IL17) and an effective treatment option for psoriatic arthritis (PsA) and ankylosing spondylitis (AS). Phase III study safety data indicate a possible risk of inflammatory bowel disease (IBD), a… read more here.
Keywords: psa; safety; ibd; secukinumab ... See more keywords

Response to Secukinumab on Synovitis using Power Doppler Ultrasound in Psoriatic Arthritis: 12-week Results from a Phase III Study, ULTIMATE.
Sign Up to like & getrecommendations! Published in 2021 at "Rheumatology"
DOI: 10.1093/rheumatology/keab628
Abstract: OBJECTIVES To investigate the dynamics of response of synovitis to interleukin (IL)-17A inhibition with secukinumab in patients with active psoriatic arthritis (PsA) using Power Doppler ultrasound. METHODS The randomised, placebo-controlled, Phase III ULTIMATE study enrolled… read more here.
Keywords: week; response; synovitis; study ... See more keywords

Paradoxical exacerbation of latent interstitial pneumonia by secukinumab in a patient with psoriasis vulgaris
Sign Up to like & getrecommendations! Published in 2019 at "British Journal of Dermatology"
DOI: 10.1111/bjd.17424
Abstract: In a recent article by Chijiwa et al.1 , anti-IL-17A antibody (secukinumab; SEC) administration was reported to reduce the elevated levels of serum KL-6 in patients with psoriasis. We have encountered a unique case of psoriasis… read more here.
Keywords: psoriasis vulgaris; paradoxical exacerbation; secukinumab; psoriasis ... See more keywords

Efficacy of secukinumab without the initial weekly loading dose in patients with chronic plaque psoriasis
Sign Up to like & getrecommendations! Published in 2019 at "British Journal of Dermatology"
DOI: 10.1111/bjd.18015
Abstract: Secukinumab is administered at the labelled dose of 300 mg at weeks 0, 1, 2, 3, 4 (loading dose) and every 4 weeks thereafter. read more here.
Keywords: efficacy secukinumab; initial weekly; without initial; secukinumab ... See more keywords

Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5‐year results from the randomized placebo‐controlled TRANSFIGURE study *
Sign Up to like & getrecommendations! Published in 2020 at "British Journal of Dermatology"
DOI: 10.1111/bjd.19262
Abstract: Secukinumab, a fully human monoclonal antibody that selectively neutralizes interleukin‐17A, a cornerstone cytokine in psoriasis, has shown long‐lasting efficacy and safety in the complete spectrum of psoriasis manifestations. read more here.
Keywords: shows high; efficacy; high sustained; secukinumab shows ... See more keywords