
Potential Energy Surfaces of Vinylogous Wolff Rearrangement: NBO Analysis and Molecular Dynamic Simulation
Sign Up to like & getrecommendations! Published in 2021 at "ChemistryOpen"
DOI: 10.1002/open.202100049
Abstract: Abstract The potential energy surfaces of the Vinylogous Wolff Rearrangement, an alternative process for the Wolff Rearrangement that takes place β,γ‐unsaturated diazoketones have been fully explored employing M062X model chemistry and in a complementary task… read more here.
Keywords: potential energy; wolff rearrangement; energy; analysis ... See more keywords
Synthesis of the hexacyclic triterpene core of the jujuboside saponins via tandem Wolff rearrangement-intramolecular ketene hetero-Diels-Alder reaction.
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron"
DOI: 10.1016/j.tet.2018.04.051
Abstract: The jujubosides are saponin natural products reported to have immunoadjuvant, anticancer, antibacterial, antifungal, and antisweet activities. The triterpene component, jujubogenin contains a unique tricyclic ketal motif comprising the DEF ring system. Herein, we describe our… read more here.
Keywords: diels alder; wolff rearrangement; tandem wolff; rearrangement intramolecular ... See more keywords

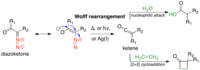
A photoinduced Wolff rearrangement/Pd-catalyzed [3+2] cycloaddition sequence: an unexpected route to tetrahydrofurans.
Sign Up to like & getrecommendations! Published in 2019 at "Chemical communications"
DOI: 10.1039/c8cc10157d
Abstract: A novel sequential reaction that combines a visible light-induced Wolff rearrangement of α-diazoketones and a Pd-catalyzed [3+2] cycloaddition of vinyl cyclopropanes with the resulting ketenes is described in this work. Selective O-allylic alkylation was observed… read more here.
Keywords: rearrangement catalyzed; catalyzed cycloaddition; photoinduced wolff; wolff rearrangement ... See more keywords

Synthesis of a versatile 1H-indene-3-carboxylate scaffold enabled by visible-light promoted Wolff rearrangement of 1-diazonaphthalen-2(1H)-ones.
Sign Up to like & getrecommendations! Published in 2023 at "Chemical communications"
DOI: 10.1039/d3cc01093g
Abstract: Herein, we have developed a sequential visible-light-promoted Wolff rearrangement of 1-diazonaphthalen-2(1H)-ones, followed by capturing the in situ generated ketene intermediates with various alcohols, producing diverse 1H-indene-3-carboxylates in moderate to good yields under mild reaction conditions.… read more here.
Keywords: rearrangement diazonaphthalen; light promoted; visible light; promoted wolff ... See more keywords

Aza-Wolff Rearrangement of N-Fluoroalkyl Triazoles to Ketenimines
Sign Up to like & getrecommendations! Published in 2023 at "Organic Chemistry Frontiers"
DOI: 10.1039/d3qo00618b
Abstract: N-Fluoroalkylated 1,2,3-triazoles underwent a microwave-heating-assisted ring opening, nitrogen molecule elimination and concomitant group rearrangement to form isolable N-fluoroalkylketenimines. This reagent-free process is characterized by a wide scope and high efficiency... read more here.
Keywords: rearrangement fluoroalkyl; fluoroalkyl triazoles; chemistry; triazoles ketenimines ... See more keywords