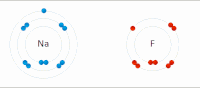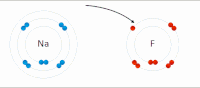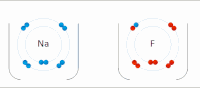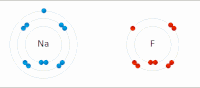
Photo from wikipedia
Theoretical studies have been carried out on the halogen bonding interaction between para substituted chlorobenzene (YC6H4Cl, Y = H, NH2, CH3, F, CN, NO2) and N(CH3)3 using ab initio MP2/aug-cc-pVDZ and DFT… Click to show full abstract
Theoretical studies have been carried out on the halogen bonding interaction between para substituted chlorobenzene (YC6H4Cl, Y = H, NH2, CH3, F, CN, NO2) and N(CH3)3 using ab initio MP2/aug-cc-pVDZ and DFT based wB97XD/6-311++G(d,p) methods. The positive electrostatic potential (VS,max) on the Cl atom and the heterolytic bond breaking enthalpy of the CCl bond have been calculated and their role on halogen bonding is discussed. The heterolytic bond breaking enthalpy of the CCl bond is proposed as a measure of the strength of the σ-hole on Cl atom. The binding strength of the complexes ranging between −6.13 kJ mol−1 and −9.29 kJ mol−1 are linearly related to the VS,max of the Cl atom and the bond breaking enthalpy of the CCl bond. In addition, energy decomposition analysis was performed on the halogen bonded complexes via symmetry adapted perturbation theory (SAPT) to predict the dominant energy component and the nature of the N···Cl interaction.
Journal Title: International Journal of Quantum Chemistry
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!