Photo from wikipedia
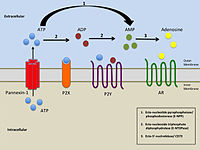
Pannexin (PANX) family proteins form large-pore channels that mediate purinergic signaling. We analyzed the cryo-EM structures of human PANX1 in lipid nanodiscs to elucidate the gating mechanism and its regulation… Click to show full abstract
Pannexin (PANX) family proteins form large-pore channels that mediate purinergic signaling. We analyzed the cryo-EM structures of human PANX1 in lipid nanodiscs to elucidate the gating mechanism and its regulation by the amino terminus in phospholipids. The wild-type channel has an amino-terminal funnel in the pore, but in the presence of the inhibitor probenecid, a cytoplasmically oriented amino terminus and phospholipids obstruct the pore. Functional analysis using whole-cell patch-clamp and oocyte voltage clamp showed that PANX1 lacking the amino terminus did not open and had a dominant negative effect on channel activity, thus confirming that the amino-terminal domain played an essential role in channel opening. These observations suggest that dynamic conformational changes in the amino terminus of human PANX1 are associated with lipid movement in and out of the pore. Moreover, the data provide insight into the gating mechanism of PANX1 and, more broadly, other large-pore channels. Description Movement of the N-terminal domain determines whether lipids obstruct the pore of the channel pannexin-1. A protein domain and lipids at the gate The large-pore, multisubunit channel pannexin-1 (PANX1) enables permeation of ions, metabolites, and second messengers, such as ATP, across the plasma membrane. Kuzuya et al. generated structures of wild-type, mutant, and pharmacologically inhibited channels by cryo–electron microscopy (see the Focus by Anderson and Thompson). Combined with electrophysiological analysis and molecular dynamics simulations, these studies showed that gating of this large-pore channel was determined by movement of the intracellular N terminus and, subsequently, the diffusion of lipids into or out of the pore. Understanding the gating of PANX-1 may lead to ways to manipulate channel activity, which plays a role in pain sensation and cancer metastasis.
Journal Title: Science Signaling
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!